A battery is an electrochemical device that converts stored chemical energy directly into electrical energy (Direct Current or DC).
It is composed of one or more electrochemical cells, each containing two electrodes—a positive cathode and a negative anode—which are separated by a conductive medium called an electrolyte.
When an external circuit is connected, a chemical reaction (a redox reaction) occurs within the battery, causing electrons to flow from the anode to the cathode through the external circuit, thereby generating an electric current.
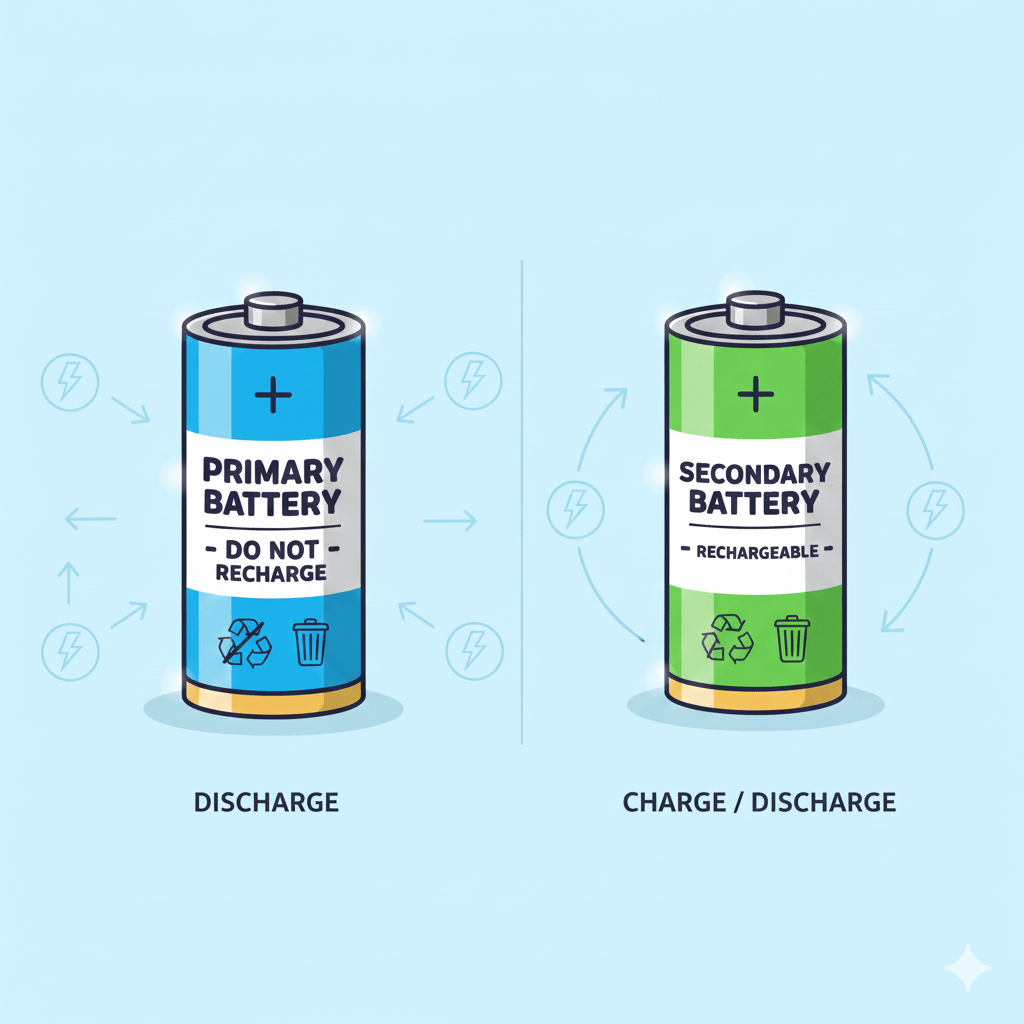
Conversely, in a rechargeable, or secondary battery, this chemical reaction can be reversed by applying an external electric current (charging), which stores the energy again as chemical potential.
The charge a battery holds refers to the amount of electrical energy it can store and deliver before being fully discharged.
This capacity is typically measured in ampere-hours (Ah) or milliampere-hours (mAh), which represents the amount of current (in Amperes or milliamperes) the battery can supply for one hour at its nominal voltage.
A higher Ah or mAh rating indicates a greater storage capacity and a longer operating time. The battery’s current energy level, relative to its maximum capacity, is called its State of Charge (SOC), often expressed as a percentage. Over time, due to chemical aging and cycling, a battery’s maximum capacity will gradually diminish, affecting its overall lifespan and ability to hold a charge.
BitcoinVersus.Tech Editor’s Note:
We volunteer daily to ensure the credibility of the information on this platform is Verifiably True.
If you would like to support to help further secure the integrity of our research initiatives, please donate here: bc1qrved9tfquym6u3age7xhmnkjs2lq8j9aulperagkuhtuk5w5c35ssfpge8
BitcoinVersus.tech is not a financial advisor. This media platform reports on financial subjects purely for informational purposes.
Leave a comment