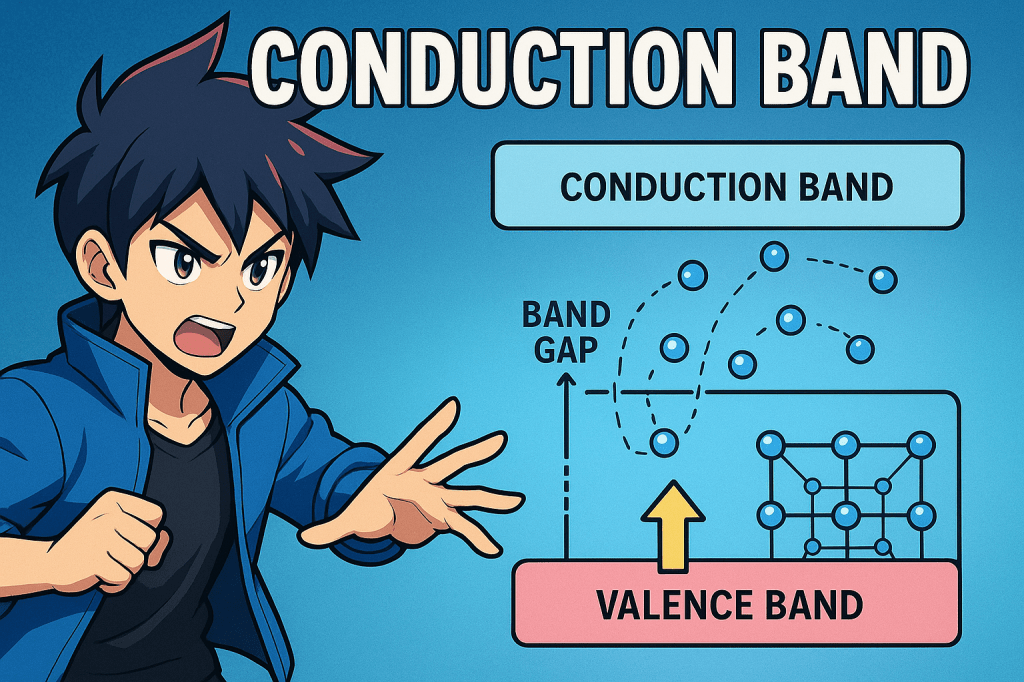
The conduction band is the range of electron energies in a solid where electrons are free to move and contribute to electrical conduction.
It lies above the valence band and is typically empty at absolute zero temperature.
When electrons gain sufficient energy to jump from the valence band to the conduction band, they become delocalized and can move freely through the crystal lattice.
These mobile electrons respond to electric fields, generating current and enabling the material to conduct electricity.
The conduction band is formed by the overlap of higher-energy atomic orbitals and contains energy states that are not occupied unless electrons are excited.
In metals, the conduction band overlaps with the valence band, allowing electrons to flow freely without needing external energy.
In semiconductors and insulators, the conduction band is separated from the valence band by a band gap, and only electrons with enough energy can cross this gap.
The number of electrons in the conduction band and their mobility directly influence a material’s conductivity.
BitcoinVersus.Tech Editor’s Note:
We volunteer daily to ensure the credibility of the information on this platform is Verifiably True.
If you would like to support to help further secure the integrity of our research initiatives, please donate here: bc1qrved9tfquym6u3age7xhmnkjs2lq8j9aulperagkuhtuk5w5c35ssfpge8
BitcoinVersus.tech is not a financial advisor. This media platform reports on financial subjects purely for informational purposes.
Leave a comment