semiconductors
-
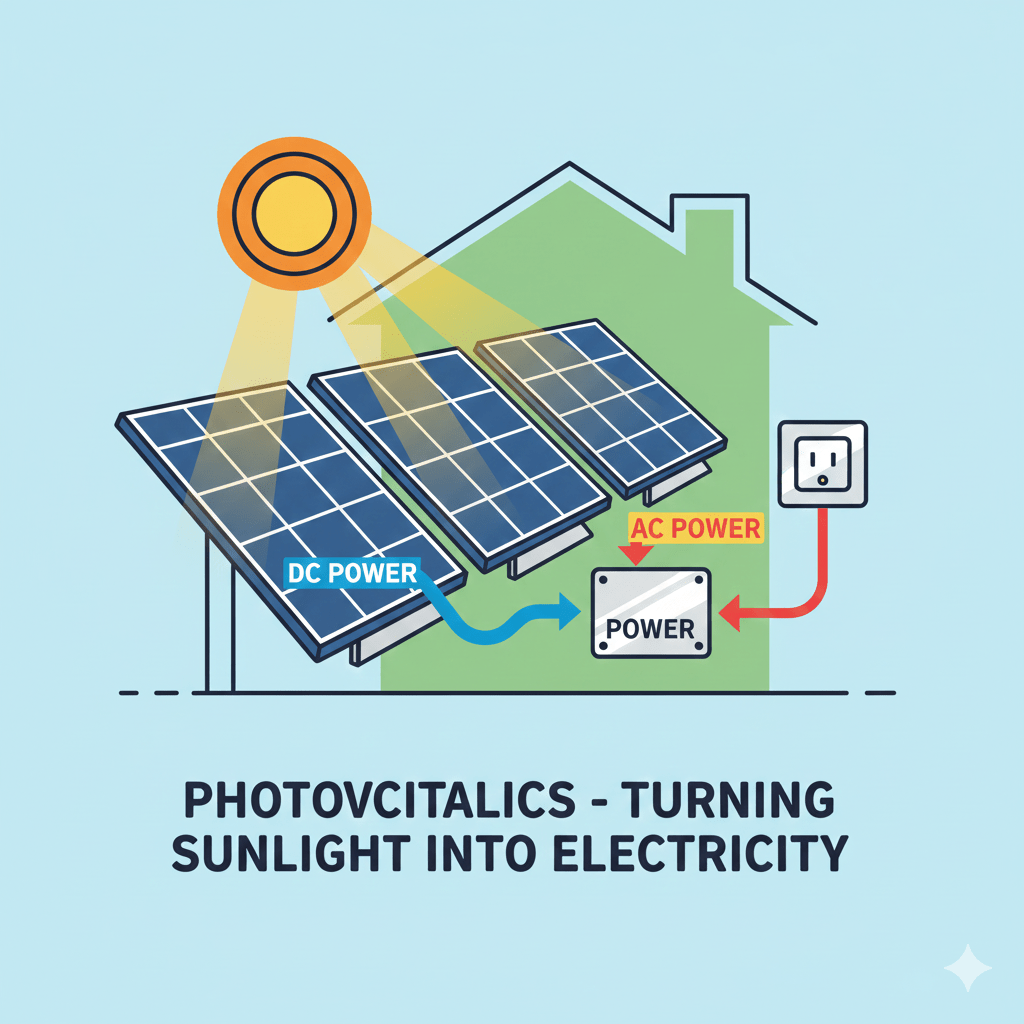
Photovoltaics is the technological principle and scientific field concerned with the direct conversion of light energy, specifically photons from sunlight, into electrical energy through the photovoltaic effect, a physical and chemical process that occurs within a class of materials known as semiconductors, most commonly crystalline silicon. The fundamental unit of this conversion is the photovoltaic cell,…
-
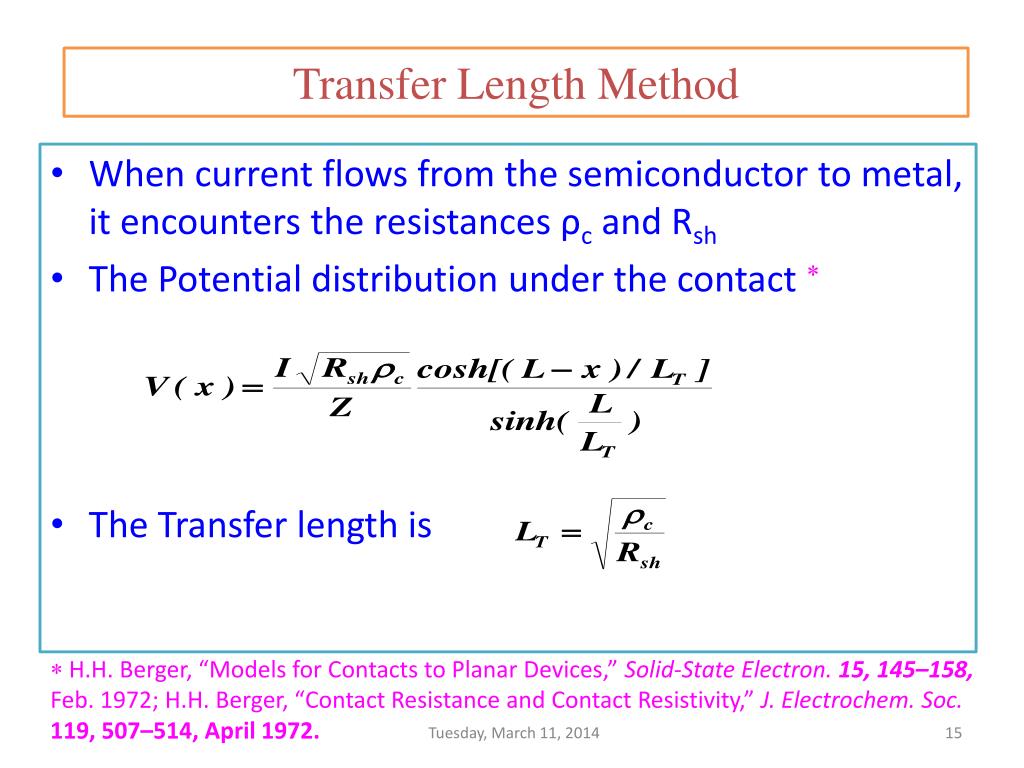
The Transfer Length Method (TLM) is a widely used technique in semiconductor physics and engineering for determining the specific contact resistivity between a metal and a semiconductor. It was developed as a response to the increasing significance of contact resistance in microelectronic devices due to device miniaturization. In practice, TLM involves depositing a series of…
-
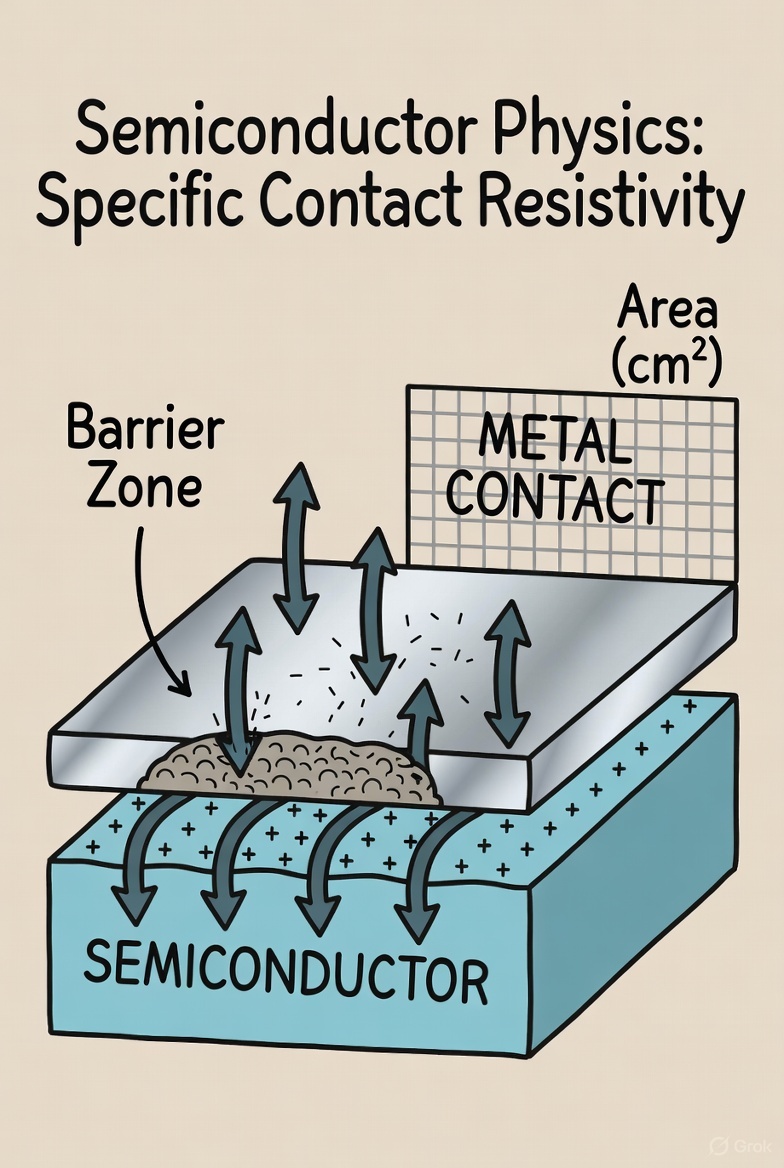
Specific contact resistivity is the fundamental measure of how effectively a metal makes an electrical connection to a semiconductor material. Think of it as a report card for the quality of the contact itself, completely separate from its physical size. Its value, given in units of Ohm-centimeters squared, tells you how much inherent resistance exists…
-

Contact resistance in semiconductor diodes refers to the parasitic electrical resistance that arises at the interfaces between the semiconductor material and the metal contacts (electrodes) that are attached to it. These metal-semiconductor interfaces are critical for injecting current into or extracting current from the active regions of the diode. The origin of this resistance is…
-
Resistance is the measurable opposition to the flow of electric current presented by a specific, complete object, such as a wire or a resistor. It is an extrinsic property, meaning its value is not fixed but is instead dictated by the physical geometry of the object. A long and narrow component will exhibit high resistance, making…
-
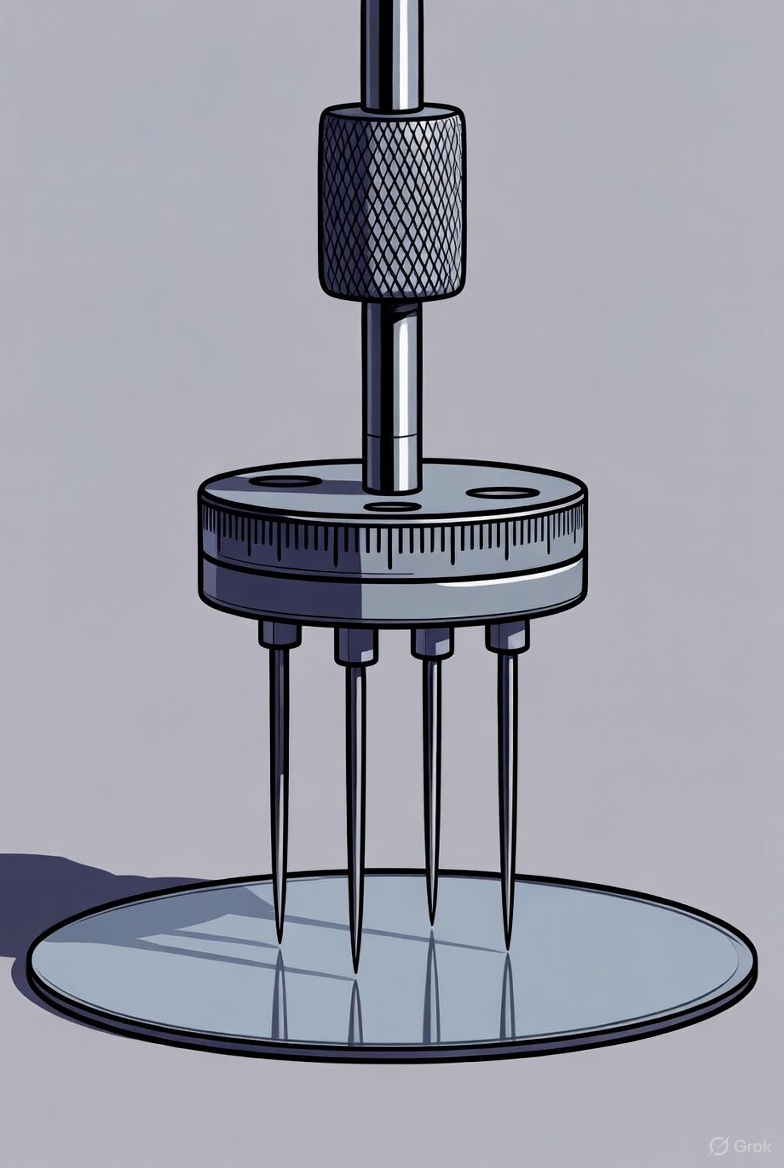
The Four-Point Probe (4PP) method is a standard, non-destructive technique used in semiconductor manufacturing and research to accurately measure the sheet resistance and subsequent resistivity of thin films, wafers, and other semiconductor materials. This technique uses a linear array of four closely spaced, collinear probes typically made of tungsten or beryllium copper, each mounted on…
-
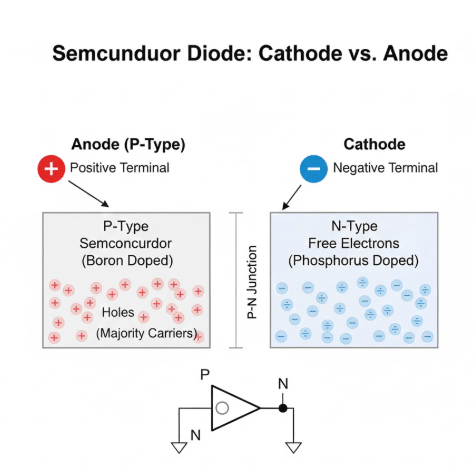
The cathode is the terminal of an electrical device through which current flows out of the device in conventional current terms (positive to negative). In a diode, the cathode is typically marked with a stripe and is the side connected to the negative voltage during forward bias. It is the terminal where electrons enter the…
-
A diode is a two-terminal semiconductor device that permits current to flow primarily in one direction, making it a fundamental component in electronics. Electrical characterization of diodes involves analyzing their current–voltage (I–V) relationship, which reveals distinct operating regions. In a diode, the anode and cathode are the two terminals that define its directionality and electrical…
-

In semiconductor technology, particularly within the foundational Metal-Oxide-Semiconductor (MOS) structure, oxide charges represent a critical class of parasitic defects—localized electric charges trapped either within the gate oxide layer (typically silicon dioxide) or at its delicate interface with the semiconductor substrate. These charges are not intentional but are inevitable byproducts of fabrication imperfections, chemical contamination, or…
-
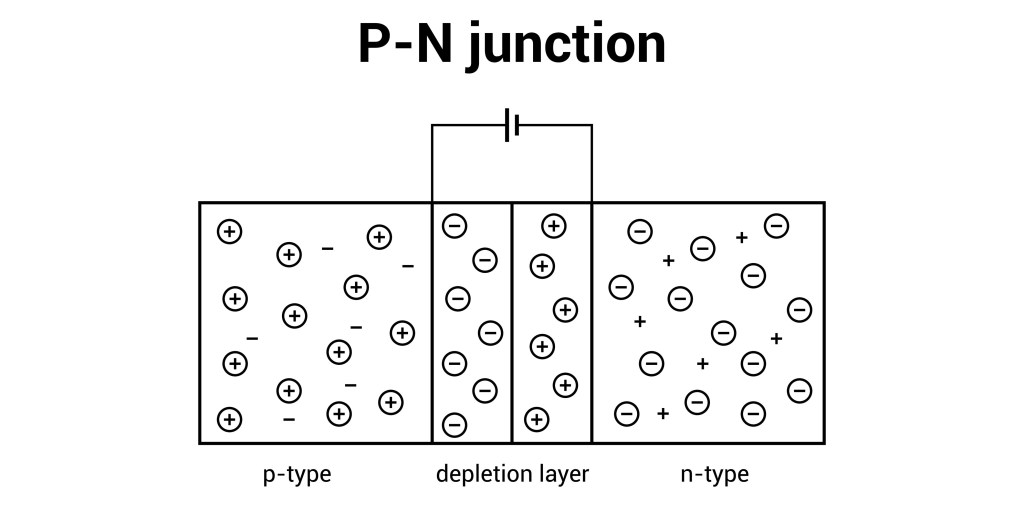
A p-n junction diode is the simplest form of a semiconductor electronic device, created by joining a p-type and an n-type semiconductor material within a single crystal. The p-type material is doped with acceptor impurities, resulting in an excess of holes (positive charge carriers), while the n-type material is doped with donor impurities, resulting in…
-

The I-V characteristics of a diode describe how the electric current flowing through the device responds to changes in the voltage applied across its terminals. In forward bias, when the positive terminal of a voltage source is connected to the diode’s anode and the negative to its cathode, the diode initially resists current flow until…
-
-
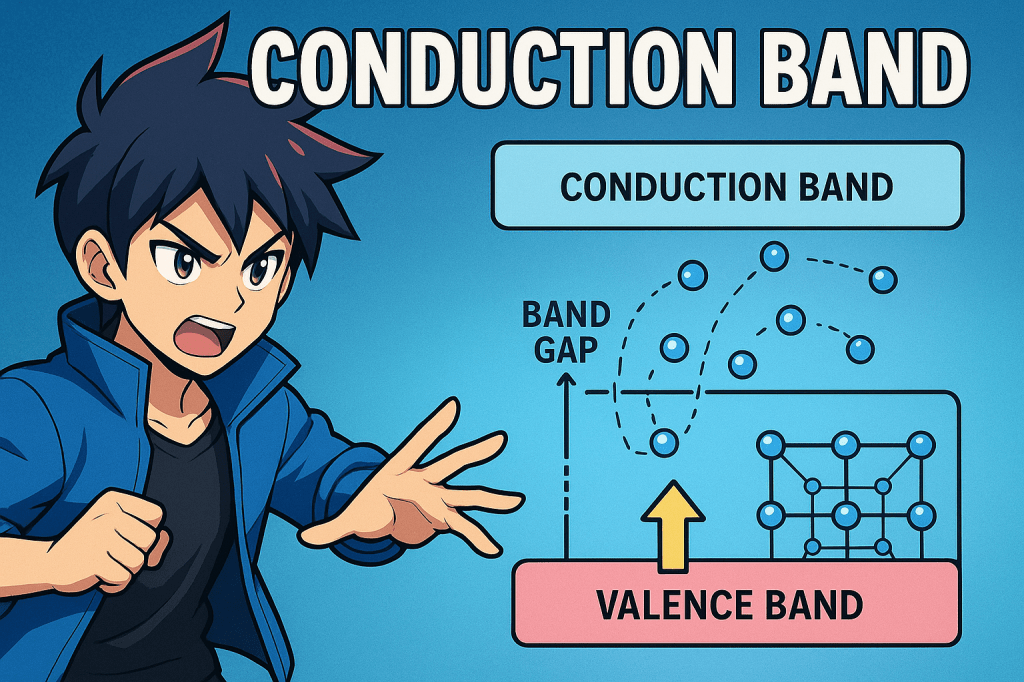
The conduction band is the range of electron energies in a solid where electrons are free to move and contribute to electrical conduction. It lies above the valence band and is typically empty at absolute zero temperature. When electrons gain sufficient energy to jump from the valence band to the conduction band, they become delocalized…
-
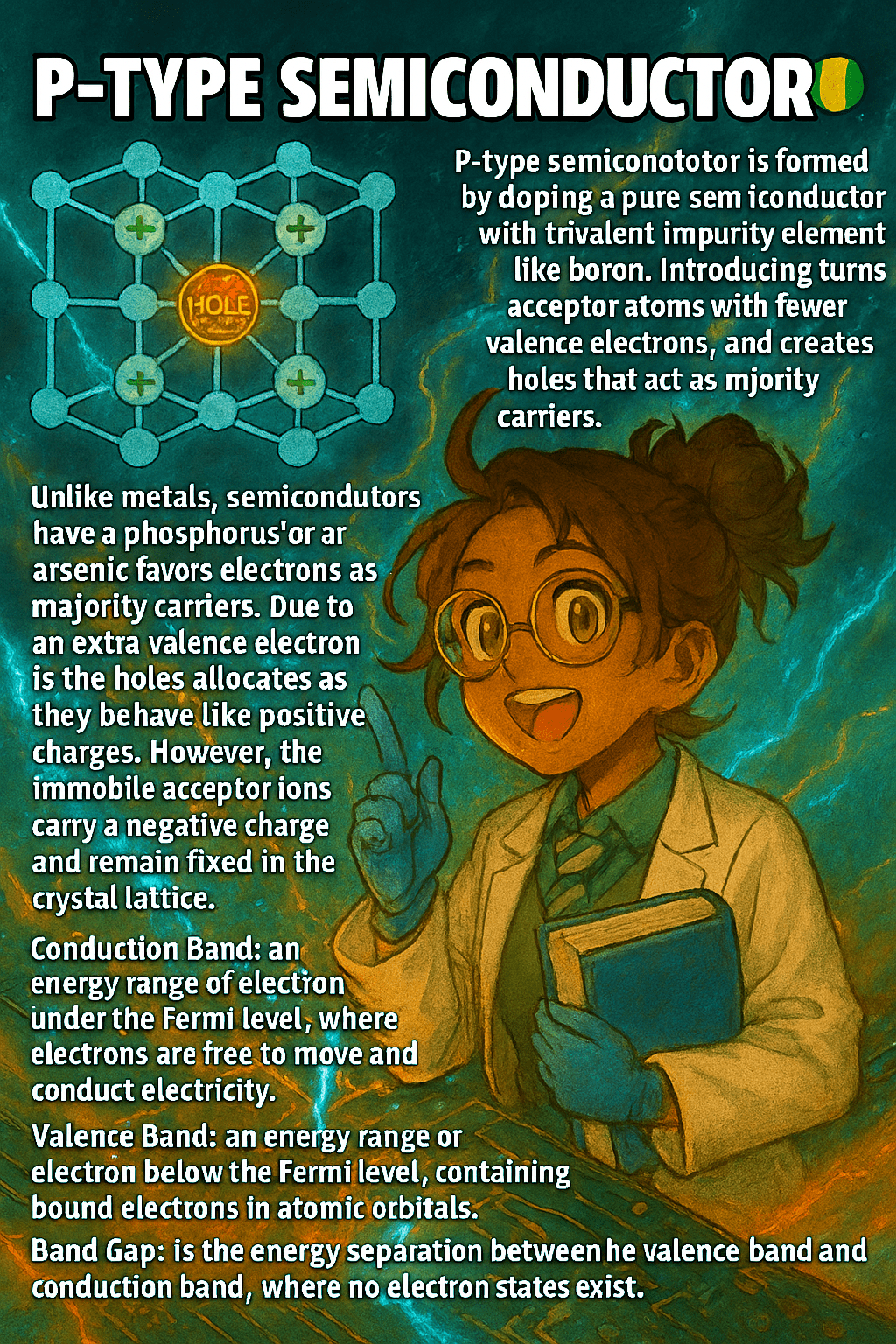
A p-type semiconductor is formed by doping a pure semiconductor, such as silicon, with trivalent elements like boron, gallium, or indium. These dopants have only three valence electrons, one fewer than silicon, which creates a vacancy or “hole” in the crystal lattice. Holes act as positive charge carriers because electrons from neighboring atoms can move…
-

The valence band is the highest range of electron energies in a solid where electrons are normally present at absolute zero temperature. These electrons are bound to atoms and participate in chemical bonding, such as covalent or metallic bonds. In crystalline solids, the valence band is formed by the overlap of atomic orbitals, creating a…
-
An n-type semiconductor is created by doping a pure semiconductor, such as silicon, with pentavalent elements like phosphorus, arsenic, or antimony. These dopants have five valence electrons, one more than silicon, and the extra electron becomes loosely bound and available for conduction. As a result, electrons become the majority charge carriers in n-type materials, while…
-

Silicon is a chemical element with atomic number 14, belonging to Group 14 of the periodic table, and it is the most widely used semiconductor material. Its electron configuration is [Ne] 3s² 3p², meaning it has four valence electrons available for bonding. In its crystalline form, silicon adopts a diamond cubic lattice structure, where each…
-

Semiconductors are materials whose electrical conductivity lies between that of conductors and insulators, and they are unique because their properties can be controlled and modified. Semiconductors are chemically diverse materials whose electrical conductivity lies between that of conductors and insulators, and their behavior can be precisely engineered. The most common elemental semiconductors are silicon (Si),…
-

Insulators are materials that resist the flow of electric current because their electrons are tightly bound to their atoms and cannot move freely. This high resistivity makes them ideal for preventing unwanted current flow and protecting users from electrical hazards. Common examples include rubber, glass, porcelain, and plastic, all of which are widely used to…
-
Resistivity is a fundamental property of materials that describes how strongly they oppose the flow of electric current. Unlike resistance, which depends on the dimensions of a conductor, resistivity is an intrinsic characteristic that remains constant for a given material under specific conditions. It is mathematically expressed as, where is the resistance, is the cross-sectional…
-

In semiconductor crystals, the atomic arrangement is defined by a combination of a Bravais lattice and a basis. The Bravais lattice provides the geometric framework—an infinite array of points arranged with translational symmetry—where each point has an identical environment. There are 14 unique Bravais lattices in three dimensions, grouped into seven crystal systems such as…
-

Transmission electron microscopy (TEM) is a powerful imaging technique that uses a beam of electrons transmitted through an ultrathin specimen to produce highly magnified images of its internal structure. TEM works by directing electrons through a sample that is typically less than 100 nanometers thick. As the electrons pass through, they interact with the atoms…
